When a Lithium Atom Forms an Li+ Ion
AnswerWhen a lithium atom forms an Li ion the lithium atom 1 gains a proton 2 gains an electron 3 loses a proton 4 loses an. Which symbol represents a particle that has the same total number of electrons as S 2- o 2- si se 2- Ar.
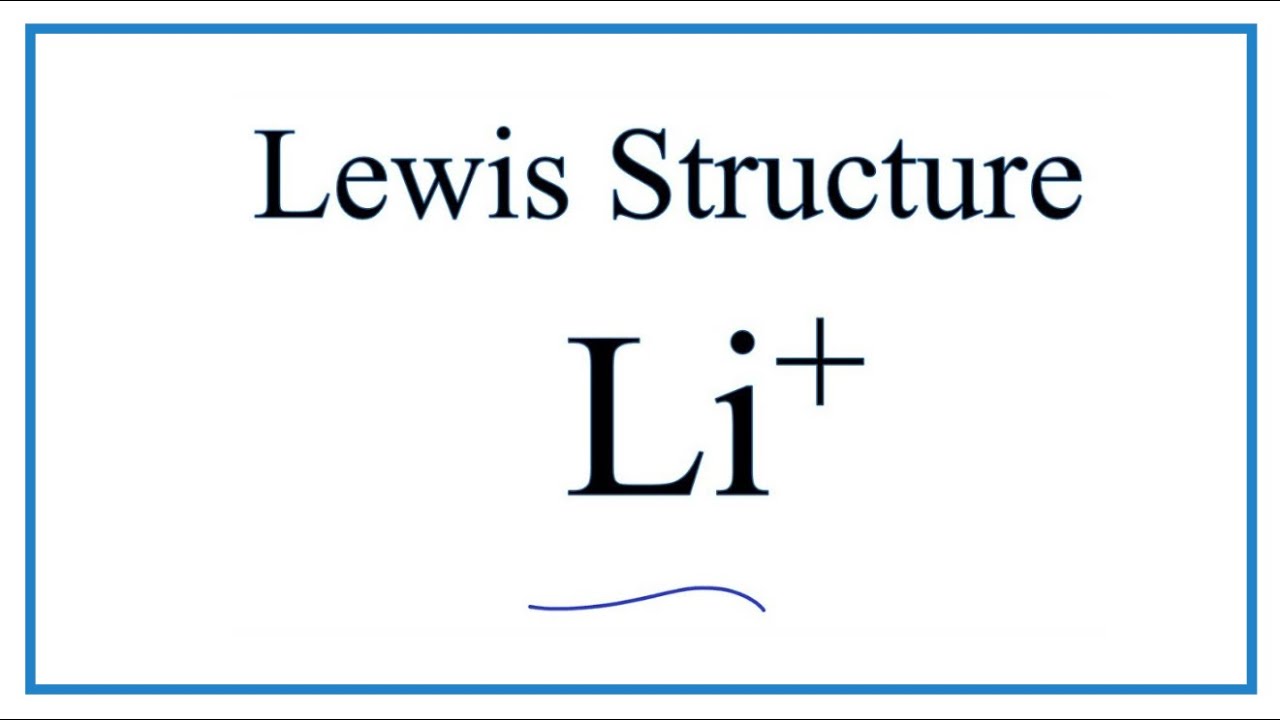
How To Draw The Li Lewis Dot Structure Youtube
The electron configuration is LI 1s² 2s¹ or He 2s¹.

. For example Li is called lithium in the names of compounds containing this ion. A simple anion obtained from a single atom is named by taking the root of the parent elements name and adding the suffix -ide. A lithium atom will form an ion by losing its single valence electron forming a positively charged ion with a charge of 1.
Hereof what is the ion of lithium. For the same reason in Li- where there are 4 electrons there is not significant difference comparing to Li. When a Lithium Li atom forms a Lithium ion Li the Lithium atom.
Oxygen likes to have two additional electrons to make it happy. Chemistry 22062019 0630 how much energy is needed to melt 5 g of ice. Lithium belongs to the family of Alkali metals whereas Beryllium belongs to the family of Alkali Earth Metals.
The lithium has achieved the stable configuration of a noble gas. What occurs when an atom loses an electron. Similarly Na is called sodium Mg2 is called magnesium and so on.
To archive stability as any noble gas element HE Then the lithium will form an electron to form a Lithium ion which is Cation LI. Lithium Li Lithium is in Group 1. Its electron configuration will be.
The amount of energy required to remove this outermost electron from the atom in gaseous state is called their Ionization enthalpy. 1 Introduction To Chemistry 2 Analyzing Data 3 Matter-properties And Changes 4 The Structure Of The Atom 5 Electrons In The Atom 6 The Periodic. Lithium 1 is a monovalent inorganic cation a monoatomic monocation and an alkali.
A lithium atom forms only one kind of ion Li. The specific latent heat of melting for water is 334000 jkg. This means that a neutral lithium atom will have a total of 3 electrons surrounding its nucleus.
It can lose one of its electrons making it an ion. 4 rows Beside above what ion is formed by lithium. The lithium atom loses one electron to form an Li ion.
3 Show answers Another question on Chemistry. The active principle in these salts is the lithium ion Li which having a smaller diameter can easily displace K and Na and even Ca2 in spite of its greater charge occupying their sites in several critical neuronal enzymes and neurotransmitter receptors. It then becomes a lie plus or the lithium cat eye on.
How is a lithium atom Li different from a lithium ion Li. Because the lithium adam has a desire to achieve this more stable noble. The atoms radius decreases and the atom becomes a.
Lithium however can form an ion Li where the. It has one. It now has more positive protons than electrons so it has an overall positive charge.
A lithium atom will form an ion by losing its single valence electron forming a positively charged ion with a charge of 1. Is lithium an atom or an ion. Each lithium atom provides one.
Dinah Zike Laurel Dingrando Nicholas Hainen Cheryl Wistrom. Lithium have the atomic number of 3. The configuration is 1s2 in terms of the electron arrangement why is this change favorable for the lithium Atom.
Now the lithium cation Li is formed when lithium loses the electron located on its outermost shell its valence electron. Lithium Oxide Two lithium Li atoms can bond with one oxygen O atom making the formula Li 2 O. In the case of lithium lithium has one valence electron.
This picture demonstrates Paulis Exclusion Principle as Li ion is much smaller than Li. Lithium from Greek. A lithium atom has 3 protons and 3 electrons.
The 3 electrons in Li cannot exist together with n1 as the 2 electrons in Li which have n1 ms12 and ms-12. In order to form ionic bonds an atom must lose electron easily to become positively charged ion or Cation. Other articles where lithium ion is discussed.
A lithium atom forms only one kind of ion Li. In order to achieve a noble gas electron configuration like helium it needs to lose this electron and typically does in the process of forming an ionic compound. Stone is a chemical element with the symbol Li and atomic number 3.
This electron is located on the second energy level in the 2s-orbital. So naturally their protonsnumber of electrons are 3. If you just say lithium its probable you are referring to an atom.
When a lithium atom forms an Li ion the lithium atom. GlencoeMcGraw-Hill School Pub Co.

Li Electron Configuration Lithium Ion Youtube
How Many Valence Electrons Will Cation Li Have Quora

Li Electron Configuration Lithium Ion Youtube

Li Electron Configuration Lithium Ion Electron Configuration Electrons Configuration
Comments
Post a Comment